◆ What is iron deficiency anemia?
The main causes of iron deficiency anemia (IDA) can be summarized into 4 categories: (1) insufficient iron intake, (2) dysfunctions in iron absorption, (3) increased physiological requirements, and (4) chronic blood loss. The causes of disease is usually an acquired factor, but there are also a few which are inherited genetic diseases; and the reasons for IDA may vary in different regions, different eras, and different social and economic background groups (see the table below).
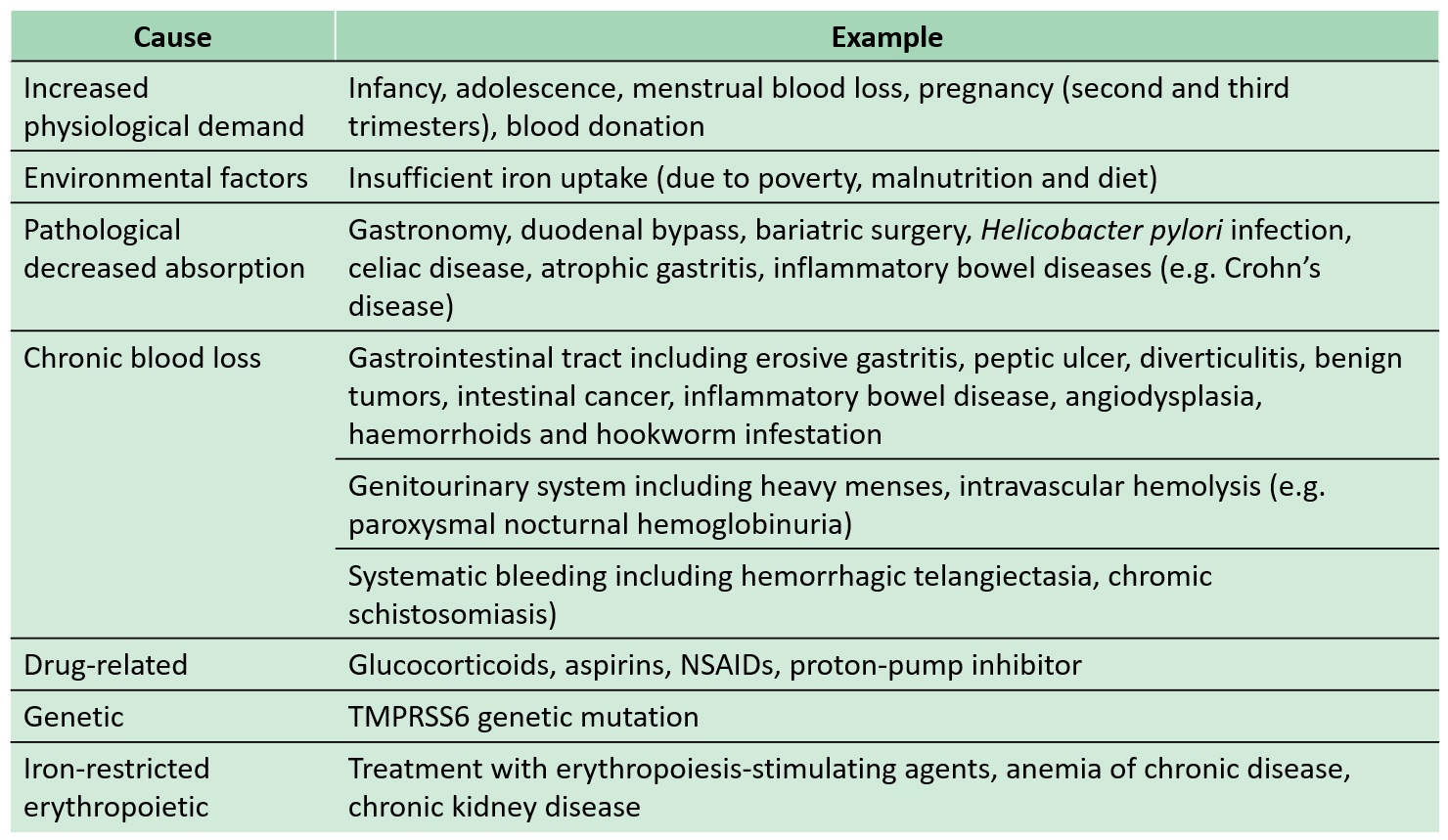
Source: Family Medicine and Primary Care Volume 31 Issue 3/Camaschella C: Iron-deficiency anemia: N Engl J Med 2015;372:1832-43.
◆ Treatment of iron deficiency anemia (IDA):
The treatment of iron deficiency anemia, in addition to correcting the cause, is to give oral iron treatment. When oral compliance is not effective or if side effects cause low medical compliance, iron injection is applied as an alternative. At present, the injection of iron is carried out through intravenous infusion. A course of treatment lasts approximately 2 months and is divided into about 10 times. Each treatment lasts about 30 to 60 minutes. This treatment course is too time-consuming for patients who do not need to stay in the hospital to undergo dialysis and occasionally causes a strong allergic reaction.
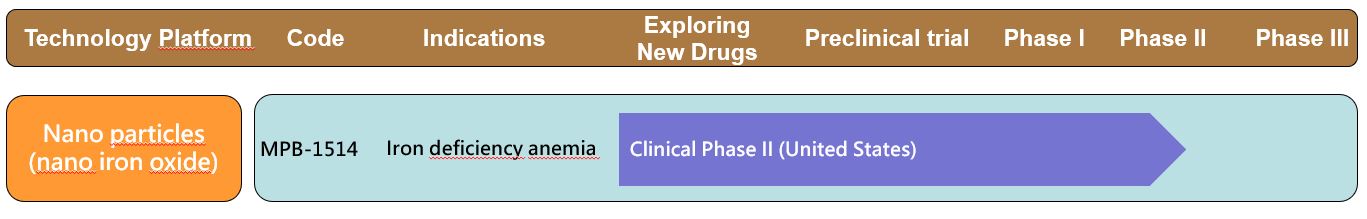
◆ MPB-1514's innovative appeals:
- Highly secure as coated with PEG with good macrophage phagocytic results.
- High doses can be injected, which can effectively increase the number of red blood cells without frequent injections, providing greater convenience and compliance with doctor's orders for patients.
- The use of commercially available products seems to continue to result in a strong hypersensitivity phenomenon. MPB-1514 must be more competitive in regards to safety. Safety is a more important consideration for users than high potency.
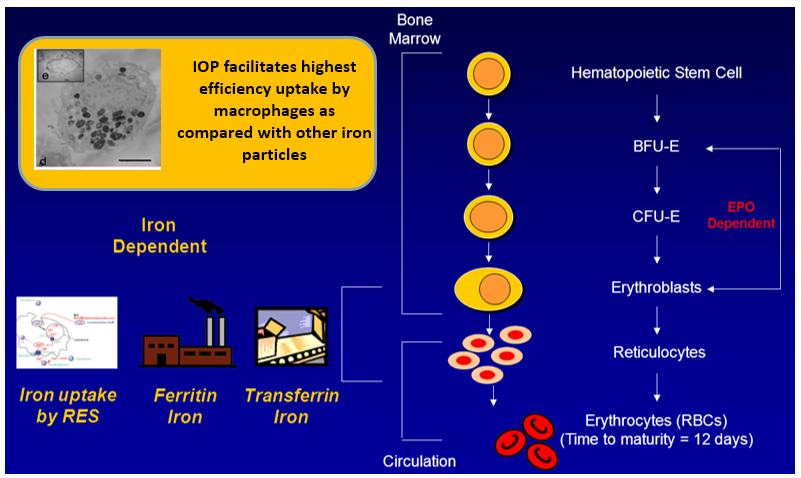
MPB-1514 has the characteristic of high macrophage phagocytic efficiency of macrophages, and it can be easily converted into transferrin (Transferrin iron) to achieve the purpose of treating iron-deficiency anemia.
◆ Clinical progress of MPB-1514:
The Phase I clinical trial has been completed at Taipei Veterans General Hospital. The Phase II clinical trial has completed the recruitment of the patients in the United States in 2020Q3.
◆ Inquiries about related clinical trials
If you need more information,please visit http://www1.cde.org.tw/ct_taiwan/archive1.html and search for the keyword "巨生"
or https://clinicaltrials.gov/ct2/home ,search for the keyword "MegaPro" in Other terms.