The MPB-1734 is an anticancer drug of a new pharmaceutical formulation. After coating commercially available paclitaxel anticancer drugs based on the company’s nanocell technology platform, animal experiments have found that in addition to the qualities of increasing drug dose while reducing low hypersensitivity during injection and the side effects of reduced immunity after injection, it has also displayed a significant inhibitory effect on malignant tumors with Paclitaxel resistance. The main development appeals are as follows:
- Applying the self-developed polymer micelle platform to coat hydrophobic anticancer drugs: This platform possesses the advantages of easy manufacturing processes, good drug coating results, and high serum stability, while being of micelle materials with excellent biocompatibility which does not cause hypersensitivity reactions and does not require to be administered with related drugs for prevention before administration, which can shorten the treatment time.
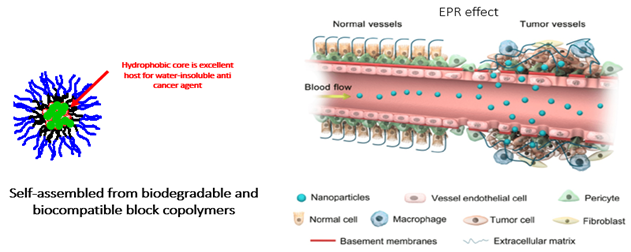
- Can be applied with immunotherapy
Through combination therapy with immunotherapy drugs, there are opportunities to increase the presentation of tumor antigens or alter the microenvironment of the tumor to increase the response of immunotherapy
◆ Clinical progress of the MPB-1734:
The first phase of the clinical trial has been approved by the US FDA, and it completed enrollment in 2024/10.
◆ Related clinical trial inquiries:
If you need more information, please go to http://www1.cde.org.tw/ct_taiwan/archive1.html and search for the keyword "Jusheng" or https://clinicaltrials.gov/ct2/home, in Other terms Search keyword "Megapro"
