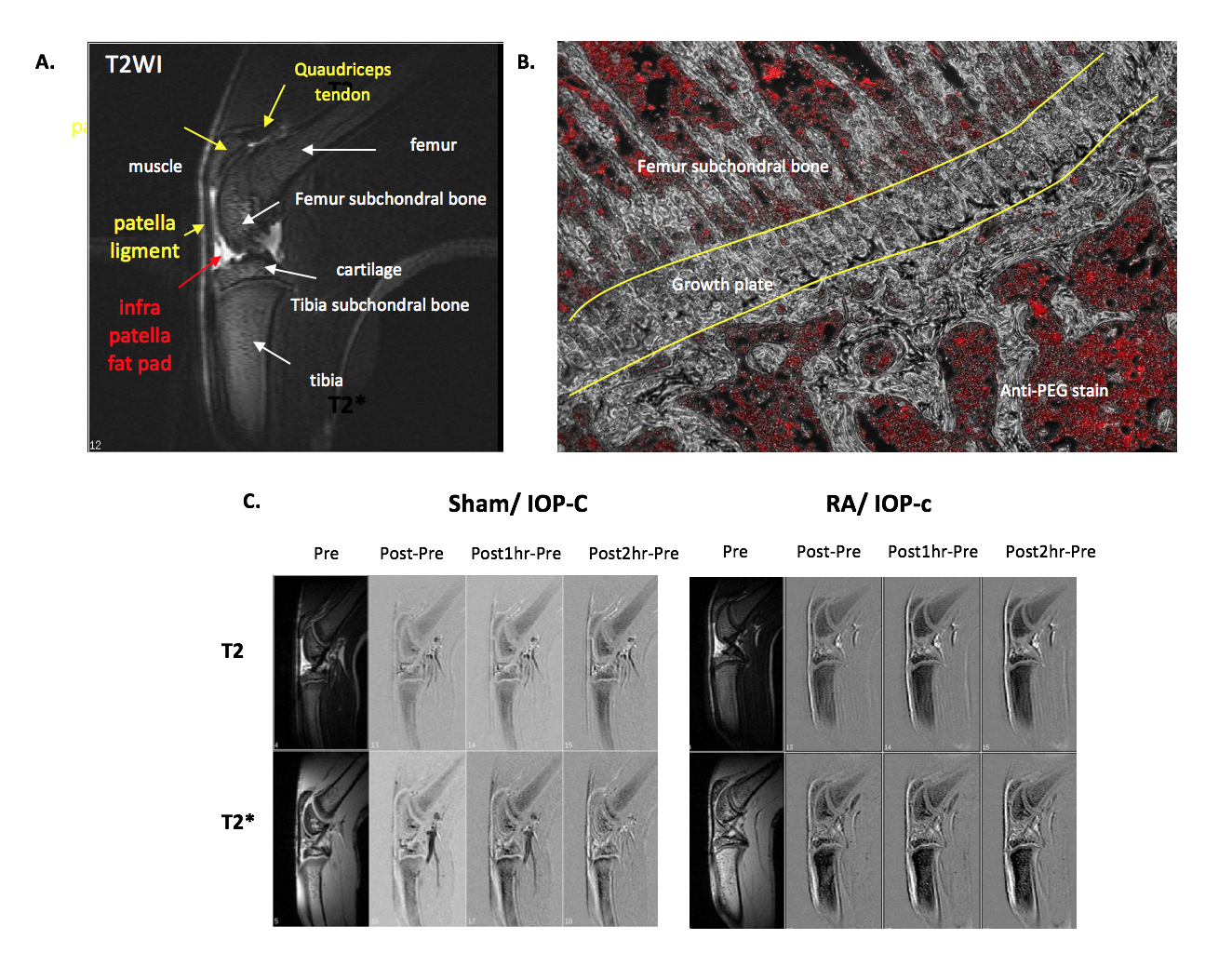
Image Calibration of Immune Cells
With the rapid development of cell biology and molecular biology technology, the research on the technology of using magnetic nanoparticles for cell identification continues to increase. Labeling cells with imaging agents, using MRI to track the distribution, movement and location of cells after cell transplantation in vivo, will allow scientists and physicians to grasp the trends and safety of cell transplantation. The MRI imaging agent currently developed by MegaPro Biomedical can also be used for immune cell labeling, including T-lymphocytes, macrophages and dendritic cells. The effect of cell phagocytosis can be carried out externally or the developer can be directly injected into the body, and by applying the higher phagocytic activity of this type of phagocytes to mark it (Figure 1).
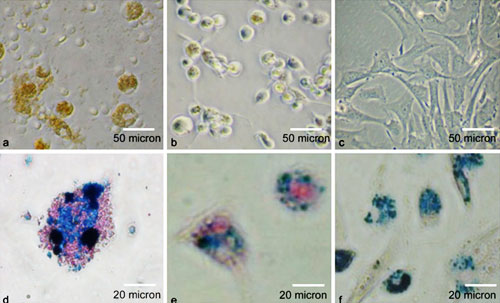
Figure 1:Light microscopy of cells labeled with ITRI-IOP. Light microscopy of a macrophages, b dendritic cells, and c MSCs cocultured with ITRI-IOP for 24 h.
After the cells are labeled with nanoparticles, coupled with high-resolution nuclear magnetic imaging equipment to track the migration of cells in the body, scientists are thus able to understand the phenomena of many immune responses, including the activation and reaction process of macrophages during tissue transplant rejections, the tracking of autoimmune diseases, and the detection of cancer cell movements in lymph nodes during metastasis. These applications will help the diagnosis of diseases and allow physicians to conduct treatment and use drugs at the most appropriate time (Figure 2).
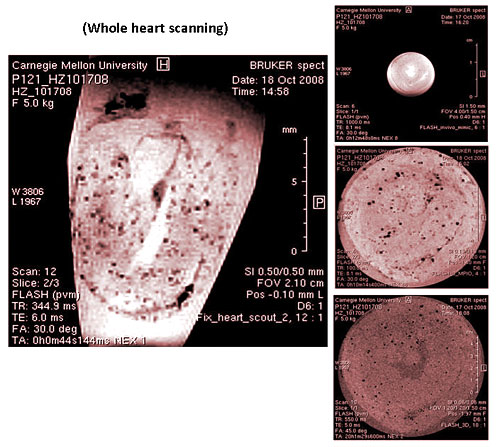
Figure 2: The process of tissue transplant rejection. Tracking phagocytic cells in vivo through a non-invasive method.